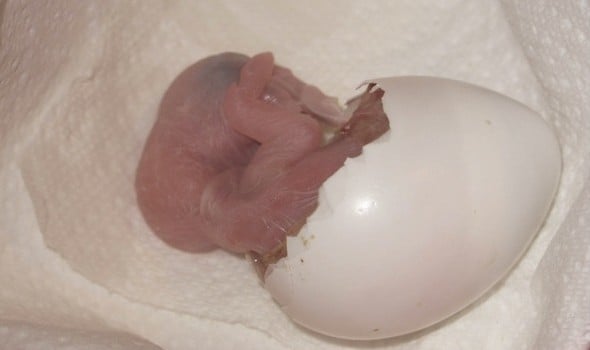
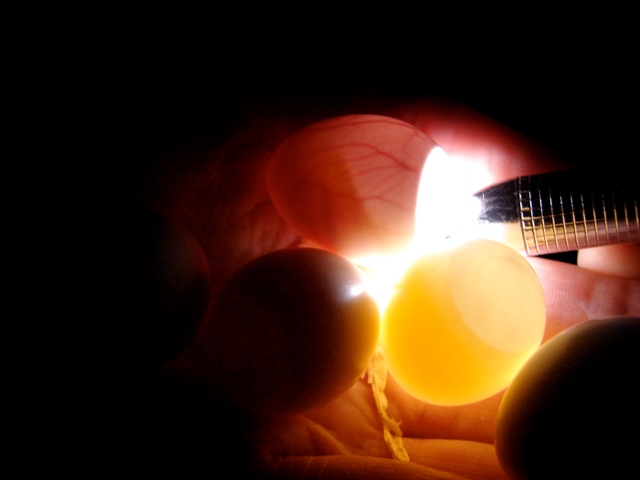
Artificial Incubation Applied to Bird Eggs
Introduction
 The egg is one of nature’s most incredible self-contained life capsules. It contains all of the balanced nutrients and, if fertilized, all of the genetic material for the creation of a new life.
The egg is one of nature’s most incredible self-contained life capsules. It contains all of the balanced nutrients and, if fertilized, all of the genetic material for the creation of a new life.
Artificial incubation goes back thousands of years when the ancient Chinese and Egyptians operated large hatcheries that were quite successful. Up until ten years ago, most of the scientific information concerning artificial incubation applied to precocial species important to the poultry industry. Presently large commercial poultry incubators fit tens of thousands of eggs at a time and, due to selection, most hatch.
The incubation of exotic bird eggs, usually in small numbers, has recently become a popular way to try and increase production but unfortunately often leads to failure. There is a desire to improve artificial incubation techniques as diets to hand-feed altricial birds right from hatch are becoming available and more breeders are confident that they can raise a baby themselves.
The topic of incubation could fill a book with many different techniques leading to a successfully hatched egg. Having the right equipment is as important as knowing the process. Temperature, humidity, ventilation, egg turning and sanitation are all important factors in the proper incubation of eggs (Brown, 1979). This paper will discuss these factors as well as some characteristics of parrot eggs, their proper collection and assistance techniques for dried out eggs.
Benefits of Artificial Incubation
The production of rare avicultural species can be significantly increased by artificial incubation. Eggs normally lost to parental neglect, predation, extremes of environmental conditions, pathogen infection and other calamities can be saved (Reininger, 1985). Many pairs lay a second clutch and in some cases triple clutch to replace eggs that are removed.
Van Der Heyden (1987) claims that artificially incubating to increase egg production is risky and that many aviculturists have lost more than they’ve gained by it. This may be due to improper techniques, poor parental nutrition and other factors that can be corrected.
Surrogate/foster parents have been successfully used to incubate and raise eggs (Gee, 1983; Harrison, 1987; Stoodley, 1984) with the same benefits as artificial incubation. Some species, such as the Monk Parakeet, make better foster parents than others. Possible disease spread, unacceptance by the foster pair and the cost (space) of maintaining them are factors which may make fostering less desirable.
HARI raised 12 babies from one of our Blue & Gold Macaw pairs which produced 18 eggs in 14 months. The first two clutches were pulled for artificial incubation within a week of being laid. Then, twice they laid again a month after we pulled three week old babies from them. Unfortunately, the demand for unrelated, parent raised, captive bred breeding stock is rather small. As a result, we have to incubate (or pull at a very young age) and hand rear most of our babies for pet stock.
 Egg Characteristics
Egg Characteristics
In different species of birds that have the same egg weight the incubation period is inversely related to the metabolic rate and the eggshell gas conductance (Rahn et al., 1974) thus similar sized eggs from different species may have different incubation periods.
The contents of fresh psittacine eggs contain slightly more solids, 19.4% (Bucher, 1983) than is typical for altricial, or semi-altricial species, which contain 17% solids (Ar and Rahn, 1980). When compared on the basis of maturity at hatching, the water fraction is significantly different between fresh altricial and precocial eggs which contain 28% solids (Ar and Rahn, 1980).
In six parrot species studied by Bucher (1983) the average incubation period was 40% longer than that of birds in general, when compared on the basis of egg mass using the data compiled by Ar and Rahn (1978), and because of the prolonged incubation periods the total embryonic energy metabolism is greater than predicted for altricial species. Bucher (1983) found that pre-piping and hatching levels of oxygen consumption are lower than in the same size eggs of precocial species.
Clutch size within the psittacines varies from one egg up to six, with two or three being average for most species (Saunders et al. 1984; Smith and Saunders, 1986;
Saunders et al. (1984) found that amoung the graminivore-frugivores, the hole-nesting psittacines produce larger clutches, have longer incubation and nestling periods than the open-nesting Columbiformes.
 Egg Collection and Record Keeping
Egg Collection and Record Keeping
Eggs left with the parents for several days of natural incubation before artificial incubation has been found to increase the hatchability (Burnham, 1983). To enhance successful copulation in wild pairs that are easily stressed, no eggs should be collected from the nest boxes until clutch completion. Some pairs do not incubate their eggs well if at all and their eggs must be pulled immediately for artificial incubation. To check for proper brooding with pairs which may leave the nest before they can be observed, one must feel the eggs (with clean hands) for warmth.
When an egg is pulled, it is labelled with a code representing the species, pair (A,B,C,…) and egg number. For instance the eighth egg from our second pair of Blue & Gold Macaws to breed is labelled BGM-B-8. The seventh egg from our third pair of Medium Sulphur-Crested Cockatoos MSC-C-7, etc. By using a soft carbon pencil every egg is identified along with approximate date of lay. Eggs left with the parent pair are not labelled but are recorded.
Rowe (1986) describes a portable incubator used to transport eggs collected in the wild and brought into captivity.
Disinfection of Eggs and Incubator Prior to Incubation
Eggs laid in dirty boxes can be covered by contaminating microorganisms from the feces or feather material of the parents or previous nestlings. There is some controversy as to benefit of cleaning, washing, treating with antibiotics or disinfecting eggs. It may be best to leave very dirty eggs in the nest but if they are to be set, some cleaning and disinfecting should probably be done. Even visually clean eggs may harbour a significant amount of bacteria or viruses on their shells and unless disinfected can spread this to other eggs in the incubator.
Eggs which have been soiled by feces can be carefully dry cleaned with fine sand paper or steel wool. Gently flick away the feces and minimize scratching the cuticle or pressing the dirt through the pores and into the egg.
Older egg submersion washing systems used by the poultry industry often served to spread and multiply bacterial contamination if wash water was too cold or re-circulated too often inactivating the disinfectant. When the wash water has a lower temperature than that of the eggs, the result is a cooling and contraction of the egg contents, which serves to draw wash water and associated bacteria through the shell pores contaminating the egg.
This temperature-differential effect has been used to introduce into eggs both water, if they have lost excessive weight (Gee, 1983), and antibiotics to control certain egg-transmitted bacterial infections (Ekperigin and McCapes, 1977). Eggs are preheated in a disinfectant solution and then immersed in a cold antibiotic solution for 2.5 minutes with no adverse effect on hatchability (Ekperigin and McCapes, 1978).
When the goal is to remove the surface contamination, all eggs should be dipped in a disinfectant solution which is always warmer than the egg, carefully wiped with a paper towel (which is dipped often to warm it up and clean it) and then quickly dried. New automated washing systems use a warm pressure wash spray containing a detergent, a hot water rinse containing a disinfectant and air blow drying (Kuhl, 1988).
Mandl et al. (1987) studied the efficacy of various disinfectants on broiler breeder eggs contaminated with salmonella. No adverse effects on hatchability were obtained when fresh eggs were soaked in 2 % solutions of aldehydes, phenols or quaternary ammonium compounds for five minutes but were significantly reduced by a 5 % solution of an iodophor compound.
Fumigation of eggs has been a common method to kill shell surface micro-organisms. Fumigation should only be done with fresh eggs, older eggs can be killed. Incubators made out of porous wood or polystyrene and others with hard to reach areas can only be effectively disinfected by fumigation. Researchers at the University of California, Davis fumigate using 1.22 ml formalin (37% formaldehyde) and 0.6 g potassium permanganate per cu ft of incubator for 20 minutes (Cutler et.al., 1985). However, egg fumigation is dangerous to the breeder who can not exhaust it directly outside after fumigation. The gas is poisonous and smells horrible. It is also inconvenient for the average breeder who has small numbers of eggs over a longer period of time.
Egg Storage
Delaying the setting of eggs allows a larger number of them to be set at the same time. This technique saves labour and simplifies the incubation and transfer of large numbers of eggs to the hatcher. It is also used by researchers studying diets, as the growth of baby chicks can begin at the same time.
Hatching eggs can be stored under appropriate conditions for up to a week prior to incubation without a significant loss in hatchability. Pre-incubation storage of cockatiel eggs at a temperature of 13°C (55°F) and relative humidity of 60% does not reduce hatchability until after four days of storage (Cutler et. al., 1985). Cutler et. al. (1985) demonstrated that bagging cockatiel eggs during the storage period appeared to increase the storage time and improve viability. Storage of the eggs in plastic bags appears to reduce the rate of dehydration and pH change due to CO2 loss from the egg.
It is recommended to gradually warm cooled eggs from storage up to room and then incubation temperatures (Haigh, 1984).
For most exotic bird breeders working with small numbers of rare and valuable eggs, the risk associated with storage is unwarranted and the technique is not really needed.
 Setting and Turning
Setting and Turning
When setting in trays, the proper orientation of the egg during incubation is with the small end pointed down. The air cell should grow at the blunt end. There is some controversy over the positioning of the egg, in a tray or on rollers. Most trays are held at 45° and rotate the eggs 90° from one side to the other, although the eggs are held in the vertical position in the tray. Rollers are able to turn the eggs completely and the eggs can be set in a more natural horizontal position. No scientific studies have compared rollers vs. trays although some breeders report better success with rollers.
Turning prevents the embryo from fusing with the eggshell membranes. If this happens the embryo will stick to the shell and development can be fatally distorted or the chick may be malpositioned for proper hatching.
Cheaper incubators are available with manual or hand turning but the savings are questionable for several reasons. Eggs should be turned at least three times a day or better still once every two hours for the first 75% of the incubation period. Manual turning may simply require turning or pulling a knob but forgetting to this will reduce hatchability. Turning eggs directly by hand has many risks including; contaminating the eggs with micro-organisms, damaging the eggs by careless turning, or twisting the embryo by turning in the same direction and forgetting to turn them.
The turning of chicken eggs can be stopped at 16 days (normal incubation period 21 days) without adversely affecting hatchability (Wilson, 1988).
 Candling and Fertility
Candling and Fertility
Candling is important to determine the approximate age of an egg and its fertility. A candling lamp should have a narrow cool beam and be portable so that eggs do not have to be handled. The translucency of white-shelled parrot eggs allows candling, smaller eggs being more translucent since they have thinner shells (Grau, 1987). Candling is best done in dim light or in the dark so that only the light passing through the shell is observed.
Fertility is observed at about 4-7 days when definite opacities will be seen, along with reddish blood vessels lining the membrane. If no development is observed after 2 weeks of incubation the egg should be removed and broken to see if any blood rings or dead embryos can be found. A blood ring is left when an embryo dies very early and the extra-embryonic blood vessels have continued to move down the yolk membrane.
 As the embryo gets bigger it is nearly opaque and very difficult to observe. The air space will become larger as the embryo develops. When the embryo is about 3/4 through its incubation, the live embryo may be detected by shinning the light into the air space and observing for motion of the embryo against the air sac membrane. The embryo is not always moving but this activity will increase and be more observable closer to the piping date which is about 2-3 days before the hatching date.
As the embryo gets bigger it is nearly opaque and very difficult to observe. The air space will become larger as the embryo develops. When the embryo is about 3/4 through its incubation, the live embryo may be detected by shinning the light into the air space and observing for motion of the embryo against the air sac membrane. The embryo is not always moving but this activity will increase and be more observable closer to the piping date which is about 2-3 days before the hatching date.
Temperature
 The rate of embryonic development is dependent on temperature. Incorrect temperature may alter the timing of the hatch and may result in incomplete absorption of the yolk.
The rate of embryonic development is dependent on temperature. Incorrect temperature may alter the timing of the hatch and may result in incomplete absorption of the yolk.
Poor quality temperature measurement and control has, in the past, been responsible for most failures in artificial incubation (Klea, 1983). Ether filled wafer thermostats are affected by barometric pressure, become inaccurate with use and even when new have a greater variability than other types of control (Klea, 1983). If the wafer bellows fails the eggs could be cooked or poisoned by the released gas. Reliable solid state thermostats are now the norm on most incubators. Add-on solid state kits are available from several manufacturers.
If an accurate thermometer, ± 1/2°C (± 1/4°F), does not come with the incubator, it should be purchased as an accessory. Kuhl Corp. have a Saybolt Thermometer available (item # 0167) with an accuracy of ±0.2°F and a range of 94 to 108°F, perfect for dry bulb incubation temperatures.
Forced-air incubators have a continuously running circulation fan keeping the conditions within uniform. Still-air incubators have no such fan which usually results in a temperature gradient increasing to the top of the incubator and closer to the heating element. Still-air models need to be operated at slightly warmer temperatures so that the optimum average temperature of the eggs is achieved. Each model may have different gradient or zone characteristics and this variability makes still-air incubators difficult to monitor and control at the required steady temperature.
Researchers at the University of California, Davis found that incubating cockatiel eggs at a temperature of 37.5°C (99.5°F) and a relative humidity of 56% produced best results. Temperatures 1.4°C higher or lower than 37.5°C produced very poor hatchability and increased the incidence of abnormalities (Cutler and Abbott, 1986). HARI’s experiences with this temperature have also been favorable, however, Stoodley (1983, 1984), a large and successful Amazon and pionus parrot breeder, incubates at the lower temperature of 36.9°C (98.5°F) with favorable results. Remote monitoring of egg temperatures during natural incubation has found lower temperatures than those commonly used for artificial incubation (Klea, 1987; Schwartz et al. 1977).
Some recommend that artificially incubated eggs be cooled once a day to recreate the natural cooling which occurs when the brooding parent leaves the nest to eat (Reininger, 1983). It was found with American Kestrel eggs that this cooling has no effect on the hatch, detrimental or helpful (Snelling, 1972). Therefore opening the incubator regularly to check fertility or for piped eggs is probably not harmful and will also bring in fresh air.
In reptiles, incubation temperature has been shown to affect the sex of the hatchlings. It appears that these eggs have the genetic potential to develop into either sex. This environmental sex determination works by producing more males at warmer incubation temperatures and more females at cooler temperatures.
This does not appear to occur in birds.
Humidity
A thermometer can be used to determine the humidity by making it into a “wet-bulb thermometer”. This is made by fitting the thermometer with a wet wick and noting its lower temperature due to evaporative cooling. The rate of evaporation is dependant on the humidity of the incubator. These wicks should be kept free of mineral buildup which can be avoided by using distilled water. The wet-bulb temperature can be converted to per cent relative humidity using standard tables for the particular dry-bulb temperature used (Table 1).
Incubators and hatchers have pans filled with water to provide humidity. Some equipment, such as the Marsh Farms Roll-X, contain quadrants in the lower tray that are flooded to provide the humidity. The total surface area of water determines the humidity and should be adjusted until the correct humidity is achieved.
Some incubators have heated water trays which can significantly increase the humidity. With these systems the correct water tray temperature needs to be set or too high a humidity may occur.
The humidity can also be controlled by the size of the intake-exhaust air vents. However proper ventilation is needed to provide O2 and remove CO2 from the incubator and restriction of this flow to increase incubator humidity is not recommended.
Low (1987) advises towards lower humidity levels and provide little or no water in the incubator. This may be true for very humid areas such as Florida but in all cases the correct humidity must be established by a combination of wet bulb readings and proper egg weight loss.
Table 1.
Percent relative humidity at different wet bulb readings and incubation temperatures.
| Relative Humidity (%) | ||||
| Wet BulbTemperatures | IncubationTemperatures | Optimum Levels | ||
| (°C) | (°F) | 36.9°C (98.5°F) | 37.5°C (99.5°F) | |
| 32.8 | 91 | 75 | 73 | ]-Hatching levels] -levels |
| 32.2 | 90 | 72 | 69 | |
| 31.7 | 89 | 68 | 66 | |
| 31.1 | 88 | 65 | 64 | |
| 30.6 | 87 | 62 | 61 | ]-Setting levels] levels] |
| 30.0 | 86 | 59 | 58 | |
| 29.4 | 85 | 56 | 55 | |
| 28.9 | 84 | 53 | 52 | |
| 28.3 | 83 | 51 | 49 | |
| 27.8 | 82 | 48 | 47 | |
| 27.2 | 81 | 46 | 44 | |
(Reinhart, 1982)
The pans of water provide growing media for pathogenic bacteria, especially in the warm environment of an incubator, and should be disinfected periodically. The use of boiled water, that has been cooled, in incubator trays will reduce the chance of unwanted organisms being introduced from the tap water.
At HARI, wet bulb readings are in the range 30.0°-30.5°C (86°-87°F) which represents a higher 58-61% relative humidity than those previously reported. With these levels (and those indicated below for the hatcher) we have experienced excellent hatchablility amoung cockatoo eggs (approx. 90%) but poorer hatchability among our macaw and Amazon eggs (approx. 70%). Random bacteriology on some of the dead-in-shell eggs found them to be free of pathogens. Malpositions seemed to be more common.Stoodley (1984) reports good results with pionus and Amazon eggs maintaining his main incubator at 53% r.h. but has several others at higher and lower r.h. levels to correct excess or insufficient loses. The Macdonald Raptor Research Centre of McGill University incubates more than 500 kestrel eggs per year with modified Marsh Farms Roll-X incubators maintained at 37.5°C and the humidity (approx. 55%) is provided by flooding one quadrant of the incubator floor (Bird, 1987).For the next breeding season (1990) HARI will set up a second incubator to separately set Amazon and macaw eggs at a lower relative humidity, probably around 55%.
Weight Loss Determinations
Nearly all weight loss is due to the diffusive transport of water vapour across the eggshell (Rahn and Ar, 1974; Ar and Rahn, 1980). The rate of water loss is determined by a species-specific water-vapour conductance, number of pores, pore structure and thickness of the shell (Ar et al.,1974; Rahn et al., 1976). Egg shell conductance of each species has evolved in close coordination with egg mass, incubation period, and microclimate of the nest (Carey, 1980).
The weight loss rate from lay to pip is linear if the eggs are exposed to the same temperature and humidity during this period (Olsen and Olsen, 1987) and can be controlled by differing the level of humidity in the incubator. If the egg is losing weight too quickly, raising the humidity will slow the rate of evaporation through the egg’s pores and conversely lowering the humidity will increase the rate of weight loss. Convection or a draft created by the incubator fan has a minimal effect on egg water loss (Weinheimer and Spotila, 1978).
Some recommend observing the size of the air-cell as a means of checking for correct weight loss or the age of the egg. This can be inaccurate due to the different shapes of eggs and variation in the amount of membrane separation and protrusion of the embryo into the air cell. For example; a moluccan cockatoo egg of unknown age was pulled and candled showing what appeared to be a large air-cell occupying about a third of the egg and it looked clear. Based on this it was thought the egg was old and infertile and was not incubated. On breakout a small one week old embryo was found and the air cell was actually small in volume as it wrapped around the egg membranes causing the embryo to protrude into what appeared to be a large air-cell. Needless to say HARI no longer uses the air-cell size or rate of size increase as an indicator of egg age or rate of weight loss.
Several formulas can be used to determine the accepted and observed rate of weight loss or overall per cent weight loss and to then correct the humidity if the observed values are off. The zoo community is aware of these formulas (Hoffman, 1987; Johnson, 1984) however private aviculturists usually do not have accurate enough scales or the propensity to work out these calculations.
From the normal incubation period (p in days), accepted mean overall fractional weight loss (the percentage of the initial egg weight lost) (fn) for that species and fresh egg weight (wf in grams) the predicted daily weight loss (dp in grams/day) for that particular egg can be calculated as follows;
- Wf x f n / p = d p
This calculated daily weight loss can be compared to the actual observed daily loss (d0) to see how close they are. The actual loss can be determined by weighing the incubating egg over a period of time (wf can be the first or fresh weight and ws is the second weight) and dividing the difference by the number of days between weighings (t) as follows;
- Wf – w s / t = d 0
The longer the time interval between egg weighing to determine d0 the more accurate the overall per cent weight loss calculated with this value will be.
If the fresh weight of the egg is not known it can be calculated from the egg size. The length (l) and breath (b) (maximum diameter) of the egg can be accurately determined with calipers. These measurements can be used in the formula worked out by Hoyt (1979) where kw is a species-specific weight coefficient. Saunders and Smith (1981) found no significant difference in kw between five species of cockatoo and determined its mean value: kw = 0.554 ± 0.015. Based on the 26 species that Hoyt (1979) examined kw has mean value of 0.548 ± 0.016 and this can be used as kw if the species-specific value is not known;
- W f = kw x l x b2
The normal incubation period (p) for most parrot eggs laid in captivity is known and this together with the observed daily weight loss (d0) and fresh weight (wf) can be used to calculate the actual observed fractional weight loss (f0) for that egg expressed in percent:
- d0 x p / wf(100) = f0
If f0 is not within the accepted range for that species then adjustments should be made to the incubator humidity. By setting up several incubators at slightly different humidities, eggs which are losing weight too rapidly or not fast enough can be moved into an incubator of higher or lower humidity for the remainder of the incubation (Stoodley, 1984).
Most larger amazon and cockatoo species lay eggs weighing 15 to 30 grams with macaw eggs weighing up to about 37 grams when freshly laid. A 15% mean fractional water loss was observed in 32 species of altricial eggs (Ar and Rahn, 1980). Based on a 26 to 28 day incubation period and this fractional loss, the daily loss calculates out in the range 0.06 to 0.19 g/day for the 15 to 30 g eggs and up to 0.22 g/day for the larger macaw eggs. An electronic balance with an accuracy of ± 0.01 g would be needed to observe such a small daily egg weight loss however if the egg is weighed over a period of a week an accuracy of ± 0.1 g may be sufficient.
The weight loss rate after piping and up to hatching may be greater than that during the “setting” period of incubation. To determine the entire incubation weight loss the egg should be weighed moments before hatching which is difficult to time correctly. Burnham (1983) took this measurement as the embryo was turning in the egg and determined that peregrine eggs lost 2.2% of the fresh egg weight between piping and hatching in a forced air hatcher set at 37.0°C and 60% humidity. This weight loss could be used as an approximate estimate for other altricial species to add to lay to pip data to determine the total fractional weight loss over the entire incubation period.
The fn loss during incubation in the eggs of seven species of terns was 14% (Rahn et al., 1976). The similar water loss amoung the terns was found in eggs with a four-fold difference in egg size, an incubation range of 21-36 days and in species which incubate their eggs at mean ambient temperatures of about 10°-28°C (Rahn et al., 1976).
The lay to pip fractional egg weight loss for successfully hatched Barn Owl eggs was significantly different between artificially incubated (37.5°C and 48% rh with 93.5% hatchability), with a 11% loss, and naturally incubated eggs which lost 14% (Marshall, 1986). Bird and Lague’s (1980) study of successfully hatched American kestrels eggs also found differences between artificial and natural incubation egg weight loss but only during about three days of the first week of incubation and with no differences in hatchability. The mean total weight loss for piped eggs was 12.1% for artificial and 16.0% for natural incubation (Bird and Lague, 1980). A low fractional egg weight loss from lay to pip of 10% has been reported for Australian kestrels (Olsen, 1987). Fentzloff (1984) reports the best hatching results in White-tailed sea eagles using 37.0°C and 64% r.h. during setting and 36.5°C and 85-90% r.h. in the hatcher, resulting in a 13-14% weight loss over the entire incubation period.
The lay to pip weight loss of eggs incubated at HARI was determined with eggs that were pulled within a few days of being laid. Eggs that were pulled after more than a week of parental incubation were not used in this weight loss data. Eggs were weighed as soon as they were pulled and again when they pip and are transferred to the hatcher.
We found differences in the average fractional weight loss of eggs from lay to piping with different species. Double Yellow-Headed Amazons had a loss of 13.4% (n=8), Blue & Gold Macaws 12.9% (n=6), Moluccan Cockatoos 9.4% (n=4) and Medium Sulphur-Crested Cockatoos 9.7% (n=18) (Appendix 1). Remember these are losses to piping and further weight reduction from piping to hatching must be added to get the weight loss for the entire incubation period. All of these eggs hatched 36 to 60 hours after piping for a total incubation period of about 28 days (Table 2).
Work with cockatiels demonstrated an egg weight loss of 9.6 % of initial egg weight at lay over the first 15 days of incubation with these eggs piping at 16 days and hatching at 18 days (Cutler and Abbott, 1986). Stoodley (1983, 1984) reports optimum hatching in amazons and pionus with a 16% weight loss.
It therefore appears that a range of fractional weight losses resulting in successful hatching occurs within species and the average value for each species falls within a range of about 12-16%.
Table 2.
The incubation periods of psittacines bred at The Hagen Avicultural Research Institute
| Species | Days |
| Blue & Gold Macaw | 28 |
| Severe Macaw | 27 |
| Citron Crested Cockatoo | 28 |
| Medium Sulphur-crested Cockatoo | 28 |
| Moluccan Cockatoo | 29 |
| Umbrella Cockatoo | 28 |
| Double Yellow-headed Amazon | 28 |
| Yellow-fronted Amazon | 28 |
Piping and Hatching
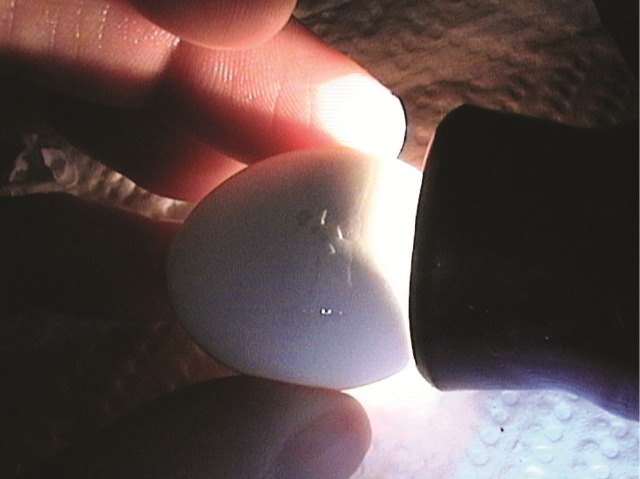 Approximately two-three days before piping the air cell gradually expands down one side of the egg. The act of shell piping usually coincides with the first breath, that is, the filling of lung and air sacs and the initial breathing movements. The air space or cell accommodates the increased volume of the embryo as lung inflation takes place. In the chicken this projected movement into the air cell continues for about nine hours during which the O2 tension in the air cell falls and the CO2 tension rises precipitously and appears to initiate the piping of the shell (Visschedijk, 1968).
Approximately two-three days before piping the air cell gradually expands down one side of the egg. The act of shell piping usually coincides with the first breath, that is, the filling of lung and air sacs and the initial breathing movements. The air space or cell accommodates the increased volume of the embryo as lung inflation takes place. In the chicken this projected movement into the air cell continues for about nine hours during which the O2 tension in the air cell falls and the CO2 tension rises precipitously and appears to initiate the piping of the shell (Visschedijk, 1968).
Once eggs pip they should be placed into a hatcher that is maintained at a slightly lower temperature, about one °C less (about two °F), and much higher humidity than the incubator. Carpenter et al. (1987) hatch eagle eggs in hatching units kept at 36.9°C dry bulb and over 32.2°C wet bulb, 71% r.h. (“as humid as possible”). We have similarly experienced maximum hatching when the hatcher has a relative humidity of greater than 80% (wet bulb about 32-33¼°C, dry bulb 36.5°C). Some incubator manufacturers make separate hatchers but these are relatively expensive units, difficult to clean and may not reach the high humidities needed to hatch some types of eggs with maximum success.
HARI has put together a still-air hatcher/brooder that is composed of an aquarium sitting in a water bath, heated by a submersible aquarium heater, all surrounded in a clear plastic pen. Using this set up (see Appendix 2 for product item numbers), we are able to achieve a high humidity, temperature controlled hatcher and also use it as a subsequent brooder.
To set up the hatcher; lay the heater horizontally into the “pen” and pass the cord between the two sections, place the two aquariums into the “pen”, fill the “pen” with warm water to about 6-7 cm, add a cup of salt to the water (this significantly reduces pathogenic micro-organism growth in the bath water), place a small hand towel over the top opening of the pen and a few paper towels in the aquariums. After allowing the heater to equalize with the water temperature, plug it in and turn it up until the pilot light comes on. Using an accurate thermometer keep turning up the heater in small increments until the bath water is 36.0°-37.5°C (97°-100°F) so that the inside of the aquariums are 35.0°-36.5°C (96°C-98.0°F). This should be set up several days before an egg is to pip. Evaporated water needs to be replaced or the heater will be exposed to the air and its glass case may explode. Clean the whole bath as needed, about every 3-4 weeks. Horizontally lay the egg, pip mark up, onto the dry paper towel inside the aquarium. Allow about 2-3 days for most eggs to hatch from the first pip. After 3 days the egg should be candled to see if the chick is stuck to the membranes and requires assistance.
HARI is now achieving better than 95% hatch from piped eggs using this setup. Previously when HARI used the hatching tray in the incubator hatching rates were only about 50% hatch from piped eggs and losses included six Amazon and cockatoo eggs.
The setup can also be used as a brooder for the first 10-14 days of a chick’s life. In this case, remove the towel from the top and lower the temperature a few degrees. The aquariums can be removed and cleaned every few days while the paper towels can be changed at each feeding. At HARI, wood shavings are used at 2 weeks of age, about the same time babies are removed from the brooder and placed in a warm room.
Researchers at the University of California, Davis place hatching eggs in a unit maintained at a hatching temperature of 36.9°C with 67% relative humidity (Cutler and Abbott, 1986). A hatchability of 81.0% of fertile cockatiel eggs was achieved by Cutler and Abbott (1986) using these levels and the previously mentioned incubation temperatures and humidity levels.
Larue and Hoffman (1981) position crane eggs in the hatcher so they are held firm, to allow the chick to rotate in the shell without rolling the egg.
A freshly hatched chick may still be attached to the allantoic sac by thin vessels which should no longer contain blood and will quickly dry and fall off.
Incubation Room
 The room where the incubator is located should be climate controlled at levels which assist the incubator rather than make it harder to meet the correct conditions for the eggs. Room temperature is best maintained between 24°-26°C (75°-80°F) and humidity between 60 to 70% r.h. Incubator heaters that are switched on frequently have a drying effect and activate humidifiers which tend to have a cooling effect. One system aggravates the other, producing poor conditions for incubation thus their use should be minimized by having stable room conditions.
The room where the incubator is located should be climate controlled at levels which assist the incubator rather than make it harder to meet the correct conditions for the eggs. Room temperature is best maintained between 24°-26°C (75°-80°F) and humidity between 60 to 70% r.h. Incubator heaters that are switched on frequently have a drying effect and activate humidifiers which tend to have a cooling effect. One system aggravates the other, producing poor conditions for incubation thus their use should be minimized by having stable room conditions.
Room temperature should not be too high or overheating and embryo mortality may occur. Some rooms are blessed with naturally ideal humidity conditions all year round, but with many it is necessary to add or remove moisture from the air. Humidifiers are especially important during dry periods such as in the winter and dehumidifiers during hot and humid summer weather. In areas where the climate is often hot and humid an incubator that has both minimum and maximum temperature and humidity controls may be the safest unit to use.
 Fertility and Hatchability
Fertility and Hatchability
Poor fertility and hatchability can be due to a number of factors including poor parental nutrition, poor parental stock (old or infirm birds), inbreeding, infection of the egg and improper incubation conditions (Flammer, 1984). Too low or too high incubation temperature and/or humidity may result in a delayed or early hatch of weak chicks with unretracted yolk sacs or death as chicks stick to the membranes or drown in excess fluid. A study of 1200 bugerigar eggs which failed to hatch found 73.3% to be infertile or clear, 14.8% as dead in shell mainly due to infections but also malposition, 11.1% because of early embryo death due to rupture of the yolk, infections or no specific cause, and about 1% due to deformed shells or lack of a yolk (Baker, 1988). Staphylococci was the most common bacteria found in infected eggs along with Streptocci, E. coli, Corynebacterium and Pseudomonas in that order of occurrence (Baker, 1988). This study shows the importance of egg sanitization to achieve maximum hatching rates.
Nutrition
Of 133 necropsies of embryos which failed to hatch or chicks which failed to survive the first month at the New York Zoological Park, 52 had lesions suggestive of a nutritional deficiency (Dolensek et al., 1979). This study found that chicks which failed to pip properly had myopathy of the hatching muscle (musculus complexus) as the most common malady. The hatchling’s muscles were enlarged from edema, hemorrhage and necrosis suggestive of vitamin E and selenium deficiency (Dolensek et al., 1979).
The importance of vitamin supplementation of seed diets was demonstrated by Roudybush (1984) who found a hatchability of 68% in cockatiels fed a vitamin supplemented diet with only 20% of fertile eggs hatching on an unsupplemented diet. The diet was a standard Cockatiel seed mix (6 parts millet, 2 parts canary seed, 1 part each sunflower, safflower, and hulled oats by weight) to which 10% hulled millet was added. The supplemented group received the vitamins coated on the hulled millet.
Thus it is important to have a sound feeding program in place which supplies all the required nutrients to a laying hen. Especially important for multiple clutching and maximum egg production is the level of calcium in the diet as egg shells are primarily calcium carbonate. The importance of calcium homeostasis is further demonstrated by its essential role in hatching muscle contraction during the hatching process (George, 1978). Most breeding pellets have adequate levels of these nutrients but if seed or bean-vege diets are being fed then a vitamin-mineral supplement formulated to meet the deficiencies of these diets must also be given daily. Mixing pellets with seeds will not provide a complete diet to birds who only select out and eat their favorite seeds and simply crush the pellets or throw them to the side.
Assisting a Piping Chick
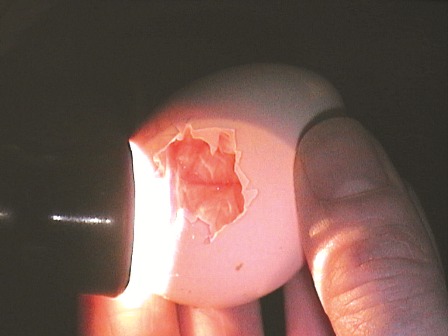 A chick which has piped but does not rotate may be saved with some delicate and risky assistance. This usually happens when the incubation humidity is too low and excessive water loss has resulted in the lack of proper lubrication of the membranes surrounding the chick. Do not mist the egg shell directly as this may drown the embryo or draw further moisture out of the egg, depending on the temperature of the egg and water.
A chick which has piped but does not rotate may be saved with some delicate and risky assistance. This usually happens when the incubation humidity is too low and excessive water loss has resulted in the lack of proper lubrication of the membranes surrounding the chick. Do not mist the egg shell directly as this may drown the embryo or draw further moisture out of the egg, depending on the temperature of the egg and water.
Only, if after three days of piping the chick has not progressed in its rotation could it be carefully helped. Remove the shell above the air cell line with a pair of tweezers and lightly wet the membrane covering the chick with a fine mist of sterile warm distilled water, saline or lactated Ringers solution. If there are no blood veins within the membrane it can be cut and the head freed. Place the egg back into the hatcher, occasionally wet the membranes covering the chick and hope the chick will free itself. Death usually occurs when assisting chicks which have not yet resorbed the blood from the vessels in the egg membranes. It appears that the chorioallantoic circulation remains functional until shortly before hatching and remains even when yolk resorbtion is complete and the embryos are vocalizing (Bucher and Barnhart, 1984). Thus the correct time to assist an egg is hard to judge and its better to wait rather than have a chick bleed to death in your hands.
Some of the birds that HARI has tried to help out had unabsorbed yolk sacs. These eggs were sometimes first incubated by the parent birds who may not have kept the eggs warm enough and thus slowed their development or they were Amazon and macaw eggs which may have not lost enough water during incubation. If you see an unabsorbed yolk sac immediately stop breaking away the shell and place the egg back into the hatcher. Hopefully the chick will free its self once the yolk sac has been absorbed. To speed up drying and prevent infection, swab some diluted tamed iodine onto a raw umbilicus or unretracted yolk sac.
Trying to assist a hatching egg can be very frustrating with most cases usually dying. All efforts should be made to have the correct incubation conditions so that eggs hatch naturally.
Summary
The best results in artificial incubation of bird eggs are achieved with; the careful collection and preparation of clean eggs; the use of a modern, automatic turning, forced air circulated incubator with reliable and steady solid state temperature controls. There are many new models of small scale incubators now available (Appendix 2). Some with advanced features are rather expensive but the hatching of just a few additional valuable parrot chicks can remunerate the increased cost.
Artificial incubation can be frustrating; it can be an art as well as a science. However loses as well as successes provide experience and information on each particular set-up and corrective measures can then be taken. Unfortunately some breeders operate on a trial and error system, waiting to see if their eggs will hatch under the conditions they have set up and only changing the conditions based on the way the eggs have died. Measuring the rate of egg weight loss and comparing it to accepted ranges can indicate if humidity changes are required before the egg is lost.
The optimum incubation temperatures for altricial bird eggs in forced air incubators appears to be between 36.9-37.5°C (98.5-99.5°F). The mean overall fractional weight loss (the percentage of the initial egg weight lost) (fn) for most altricial species is in the range 12-16% with the freshly laid to pip loss in the range 10-14%. The optimum fn for individual species may fall at either end of these ranges. It appears the level of incubator relative humidity needed to achieve maximum hatchability in some species is higher than those previously documented. More controlled scientific studies are needed to compare incubator relative humidity, egg weight loss and hatchability.
Once eggs have piped they should be moved to a hatcher which has a much higher humidity and slightly lower temperature than the incubator. Hatching takes about two days and then the laborious task of feeding a blind, helpless altricial chick can begin.
By Mark Hagen, M.Ag.
Director of Research
Original Title:
Artificial Incubation Applied to Small Numbers of Altricial Bird Eggs
References
AR. A., C.V. PAGANELLI, R.B. REEVES, D.G. GREENE, and H. RAHN. (1974).
The avian egg: water vapor conductance, shell thickness, and functional pore area.
Condor 76:153-158.
AR, A. and H. RAHN. (1978). Interdependence of gas conductance, incubation
length and weight of the avian egg. In: Respiratiory Function in Birds, Adult and
Embryonic, ed J. Piiper. Springer Verlag, Berlin pp 227-238.
AR, A. and H. RAHN. (1980). Water in the avian egg: overall budget of incubation.
American Zoologist 20:373-384.
BAKER, J. R. (1988). Poor reproductive performance in exhibition budgerigars: A
study of eggs which fail to hatch. Journal of Small Animal Practice 29:565-571.
BIRD, D.M. (1987). Captive Breeding Small Falcons. In: Raptor Management
Techniques Manual, eds. B.A. Giron Pendleton, B.A. Millsap, K.W. Cline, and D.M.
Bird. National Wildlife Federation ,Washington, D.C. pp 364-367.
BIRD, D. M. and LAQUE, P. C. (1980). Fertility, egg weight loss, hatchability, and
fledgling success in replacement clutches of captive American Kestrels. Canadian
Journal of Zoology 60:80-88.
BROWN, A.F. (1979). The Incubation Book. Saiga Pub. Co. Surrey.
BUCHER, T.L. (1983). Parrot Eggs, embryos and nestlings: patterns and energetics
of growth and development. Physiological Zoology 56:465-483.
BUCHER, T.L. and M. C. BARNHART (1984). Varied egg gas conductance, air cell gas
tensions and development in Agapornis Roseicollis. Respiration Physiology 55:277-289.
BURNHAM, W. (1983). Artificial Incubation of falcon eggs. J. Wildlife Management 47:158-168.
CAREY, C. (1980). Introduction to the Symposium: Physiology of the Avian Egg.
American Zoologist 20:325-327.
CARPENTER, J.W., R.R. GABEL and S.N. WIEMEYER. (1987). Captive Breeding Eagles.
In: Raptor Management Techniques Manual, eds. B.A. Giron Pendleton, B.A. Millsap,
K.W. Cline, and D.M. Bird. National Wildlife Federation, Washington, D.C. pp 350-355.
CUTLER, B.A., T.E. ROUDYBUSH and K.D. SHANNON. (1985). Viability of Cockatiel
(Nymphicus hollandicus) eggs stored up to ten days under several conditions. In: 34th
Western Poultry Disease Conference pp 104-106.
CUTLER, B.A. and U.K. ABBOTT. (1986). Effects of temperature on the hatchability
of artificially incubated cockatiel eggs (Nymphicus hollandicus). In: 35th Western
Poultry Disease Conference pp 32-35.
DOLENSEK, E. P., C. SHEPPARD and A. HERRON. (1979). Hatching problems in exotic
birds. American Association of Zoo Veterinarians Annual Conference Proceedings pp 63-64.
EKPERIGIN, H. E. and R. H. McCAPES. (1977). Preincubation dipping of turkey hatching eggs I. Effect of shell treatment on amount and variability of fluid intake. Avian Diseases 21:596-604.
EKPERIGIN, H. E. and R. H. McCAPES. (1978). Preincubation dipping of turkey
hatching eggs II. Effect of shell treatment on hatchability. Avian Diseases 22:288-295.
FENTZLOFF, C. (1984). Breeding, artificial incubation and release of White-tailed sea
eagles. International Zoo Yearbook 23:18-35.
EVANS, M. P. (1988). Incubator Problem. Parrot Society 22(11):357-358.
FLAMMER, K. (1984). Hatching problems in Psittacine birds. American Federation of
Aviculture Veterinary Seminar Proceedings. pp 1-7.
GEE, G. F. (1983). Crane Reproductive Physiology and Conservation. Zoo Biology 2:199-213.
GEORGE, J. C. (1978). The mechanism and physiology of hatching in birds. Pavo (special volume) 16:179-192.
GRAU, C. R. (1987). Fertility in bird eggs. Exotic Bird Report, Avian Sciences, UC Davis 7:6-9.
HAIGH, R. (1984). The breeding and artificial incubation of hawks, buzzards and falcons.
International Zoo Yearbook 23 pp 51-58.
HARRISON, G. (1987). Reproductive Medicine. In: Clinical Avian Medicine and Surgery;
including aviculture. Eds. G. J. Harrison and L. R. Harrison. W. B. Saunders Co. pp 620-633.
HECK, W. R. and KONKEL, D. (1983). Incubation and Rearing. In: Falcon Propagation:
A manual on Captive Breeding. Eds J. D. Weaver and T. J. Cade. The Peregrine Fund, Ithaca, New York.
HOFFMAN, K. (1987). Egg weight loss during incubation. Animal Keepers Forum 14:188-190.
HOYT, D. F. (1979). Practical methods of estimating volume and fresh weight of bird eggs.
The Auk 96:73-77.
JOHNSON, R. (1984). Management of artificially incubated bird eggs by weight loss.
American Association of Zoo Parks and Aquariums Regional Proceedings pp 199-208.
KLEA, J.A. (1983). Artificial incubation problems and solutions. International Foundation
for the Conservation of Birds Symposium pp 223-237.
KLEA, J.A. (1987). A technique for learning egg temperatures during natural incubation.
International Foundation for the Conservation of Birds Symposium pp 517-540.
KUHL, H. Y. (1988). Techniques in washing hatching eggs. Zootecnica 2(3):16-17.
LARUE, C. and HOFFMAN, K. (1981). Artificial incubation and hand-rearing of cranes. International Zoo Yearbook 21:215-217.
LOW, R. (1987). Hand-Rearing Parrots and Other Birds. Blandford Press, Dorset, U.K.
MANDEL, J., HAFEZ, H.M., WOERNLE, H. and KOSTERS, J. (1987). (Efficacy of disinfection methods for broiler breeder eggs contaminated with Salmonella.) Archive fur Geflugelkunde 51:16-21.
MARSHALL, J. D., HAGER C. H., and McKEE, G. (1986). The barn owl egg: weight loss characters, fresh weight prediction and incubation period. Raptor Research 20: 108-112.
OLSEN, P. D. and OLSEN, J. (1987). Egg weight loss during incubation in captive Australian Kestrels Falco cenhroides and Brown Goshawks Accipiter fasciatus. Emu 87:196-199.
RAHN, H. and A. AR. (1974). The avian egg: incubation time and water loss. Condor 76:147-152.
RAHN, H., C. V. PAGANELLI and A. AR. (1974). The avian egg: Air-cell gas tension, metabolism and incubation time. Respiration Physiology 22:297-309.
RAHN, H., C. V. PAGANELLI, I. C. T. NISBET, and G. C. WHITTOW (1976). Regulation of incubation water loss in eggs of seven species of terns. Physiological Zoology 49:245-259.
REINHART, B.S. (1982). Incubation. Ontario Ministry of Agriculture and Food, Publication # 234.
REININGER, K. (1983). Artificial incubation of bird eggs. Animal Keepers Forum 10:418-423.
REININGER, K. (1985). Artificial incubation of bird eggs: update. American Association of Zoo Parks and Aquarium Regional Proceedings pp 427-435
ROUDYBUSH, T. (1984). Care of breeding birds and eggs for production of chicks for hand rearing. American Federation of Aviculture Veterinary Seminar Proceedings pp 67-71.
ROWE, B. E. (1986). A unit for the transportation of developing eggs. International Zoo Yearbook 24/25:306-310.
SAUNDERS, D.A. and G.T. SMITH. (1981). Egg dimensions and egg weight loss during incubation in five species of cockatoo, and the use of measurements to determine the stage of incubation of birds’ eggs. Australian Wildlife Research 8:411-419.
SAUNDERS, D.A., G.T. SMITH and N.A. CAMPBELL. (1984). The relationship between body weight, egg weight, incubation period and nest site in the Psittaciformes, Falconiformes, Strigiformes and Columbiformes. Australian Journal of Zoology 32:57-65.
SCHWARTZ, A., J.D. WEAVER, N.R. SCOTT and T.J. CADE. (1977). Measuring the temperature of eggs during incubation under captive Falcons. Journal of Wildlife Management 41:12-17.
SMITH, G.T. and D.A. SAUNDERS. (1986). Clutch size and productivity in three sympatric species of cockatoo (Psittaciformes) in the South-West of Western Australia. Australian Wildlife Research 13:275-285.
SNELLING, J. (1972). Effects of incubation temperature and periodic cooling on artificially incubated American Kestrel eggs. In: Special Conference on Captivity Breeding of Raptors Part B Incubation, Natural and Artificial ed. R. R. Olendorff. Raptor Research Supplement 6:B17-B20.
STOODLEY, J. and P. STOODLEY. (1983). Parrot Production Incorporating Incubation. Bezels Pub. Portsmouth, England.
STOODLEY, J. and P. STOODLEY. (1984). Pionus Parrots. Bezels Pub. Portsmouth, England.
Van Der HEYDEN, N. (1987). Artificial incubation. Bird World 9(5):62-65.
VISSCHEDIJK, A. H. J. (1968). The airspace and embryonic respiration. I. The pattern of gaseous exchange in the fertile egg during the closing states of incubation. British Poultry Science 9:173-184.
WEINHEIMER, C. A. and SPOTILA, J. R. (1978). Shell resistance and evporative water loss from bird eggs: Effects of wind speed and egg size. American Zoologist 18:636. (Abstr.)
WILSON, H. R. and R. F. WILMERING. (1988). Hatchability as affected by egg turning in high density plastic egg flats during the last half of incubation. Poultry Science 67:685-688.
Appendix 1
Incubation periods and egg weight loss to pip for successfully hatched eggs.
Blue & Gold Macaw (n=6)
| Species | Near Fresh wt.(g) |
Loss to pip wt.(g) |
Days of wt.loss |
Days to pip | % loss to pip |
| BGM A 3 | 31.40 | 28.32 | 20 | 26 | 12.8% |
| BGM A 4 | 30.04 | 27.64 | 16 | 26 | 13.0% |
| BGM C 1 | 36.67 | 33.50 | 21 | 26 | 10.7% |
| BGM D 3 | 31.45 | 27.43 | 23 | 26 | 14.4% |
| BGM D 7 | 28.06 | 25.84 | 14 | 26 | 14.7% |
| BGM E 1 | 32.38 | 30.03 | 16 | 26 | 11.6% |
| Avg 12.9% | |||||
Double Yellow Headed Amazon (n=8)
| Species | Near Fresh wt.(g) |
Loss to pip wt.(g) |
Days of wt.loss |
Days to pip | % loss to pip |
| DYA A 1 | 20.36 | 18.12 | 23 | 26 | 12.4% |
| DYA A 3 | 20.17 | 18.41 | 22 | 26 | 10.3% |
| DYA A 4 | 19.52 | 16.62 | 22 | 26 | 17.6% |
| DYA A 12 | 19.90 | 18.15 | 16 | 26 | 14.3% |
| DYA A 13 | 19.43 | 17.44 | 19 | 26 | 14.0% |
| DYA A 18 | 16.40 | 14.86 | 19 | 26 | 12.8% |
| DYA B 9 | 19.33 | 17.33 | 19 | 26 | 14.2% |
| DYA B 10 | 21.82 | 19.91 | 19 | 12.0% | |
| Avg 13.4% | |||||
Moluccan Cockatoo (n=4)
| Species | Near Fresh wt.(g) |
Loss to pip wt.(g) |
Days of wt.loss |
Days to pip | % loss to pip |
| MLC B 5 | 34.14 | 31.38 | 27 | 27 | 8.1% |
| MLC B 6 | 30.72 | 28.65 | 22 | 26 | 8.2% |
| MLC B 7 | 29.48 | 26.55 | 26 | 26 | 9.9% |
| MLC D 4 | 26.23 | 24.06 | 19 | 26 | 11.3% |
| Avg 9.4% | |||||
Medium-sulphur Crested Cockatoo (n=18)
| Species | Near Fresh wt.(g) |
Loss to pip wt.(g) |
Days of wt.loss |
Days to pip | % loss to pip |
| MSC A 5 | 24.51 | 21.97 | 26 | 26 | 10.4% |
| MSC A 6 | 26.71 | 24.46 | 23 | 26 | 9.5% |
| MSC A 7 | 27.22 | 23.68 | 26 | 26 | 13.0% |
| MSC A 13 | 26.25 | 23.25 | 26 | 26 | 11.4% |
| MSC A 14 | 26.02 | 23.02 | 26 | 26 | 11.5% |
| MSC B 4 | 24.09 | 21.77 | 26 | 26 | 9.6% |
| MSC B 5 | 23.15 | 21.31 | 25 | 26 | 8.3% |
| MSC B 7 | 22.08 | 20.92 | 15 | 26 | 9.1% |
| MSC B 8 | 21.02 | 19.91 | 14 | 26 | 8.1% |
| MSC B 9 | 24.02 | 22.74 | 22 | 26 | 6.3% |
| MSC B 12 | 23.97 | 21.97 | 24 | 26 | 9.0% |
| MSC B 13 | 21.26 | 19.47 | 26 | 26 | 8.4% |
| MSC B 14 | 25.18 | 22.96 | 26 | 26 | 8.8% |
| MSC B 15 | 21.33 | 19.98 | 20 | 26 | 8.2% |
| MSC B 16 | 23.55 | 21.66 | 18 | 26 | 11.6% |
| MSC B 20 | 24.29 | 22.21 | 24 | 26 | 9.3% |
| MSC B 22 | 23.64 | 21.25 | 23 | 26 | 11.4% |
| MSC C 7 | 24.05 | 21.70 | 25 | 26 | 10.2% |
| Avg 9.7% | |||||
Appendix 2 –
Small Sized Incubators
Accurate Balances
Distributors Addresses
Incubators:
All of these models are forced air circulated incubators. Some of the cabinet units have hatching trays at the bottom of the incubator but these may not provide the proper environment for maximum hatching rates. Most of the units made overseas run on 220 V, but can be connected by your electrician. To import units from overseas requires more paper work and takes several months to ship by sea.
Format:
- Manufacturer (Distributer)
- Model: Counter top dome or Cabinet type, Manual or Automatic turning, Thermal Wafer or Solid State temperature control, Automatic humidity and/or cooling control, Grid, Egg Rollers or tray egg holders, Maximum capacity of 3.0-3.5 cm diameter eggs (referred to as pheasant size which is similar to the average parrot egg)
- Cost
- A.B. Newlife 25 MkII: Dome, Auto, Solid state, Auto. humidity, 3 small trays, 25 eggs, $855. Can. landed.
- A.B. Newlife 75 MkII: Dome, Auto, Solid state, Auto. humidity, 3 med. trays, 75 eggs, $1000. Can. landed.
- A.B. Multilife 290: Cabinet, Auto, Solid state, Auto. humidity, 3 lg. trays, 290 eggs, $2740. Can. landed.
Formed in 1986 to produce and to update the designs introduced by Dr. Anderson-Brown. Good quality but expensive to import. Their egg trays/cradles come in four different sizes which can mixed as inserts, therefore allowing most sizes of eggs to be incubated at the same time. They produce a “Humidistat” which automatically adds water onto an absorbent pad when an increase in humidity is required (note that it can not reduce humidity below ambient). The “Humidistat” can be bought separately and added to any small incubator.
ARA Professional Incubator
- ARA Professional Incubator: Egg Cap. 70 Amazon or 50 Macaw, Table Top, Auto Turn, Auto Temp, Solid State, Egg Tray $2100. US. (exludes local taxes & delivery).
- Octagon 20: Egg Cap. 20-40, Table top, Auto Turn, Auto Temp, Solid State, Egg Tray $283. US.
- Ocatagon 100: Egg Cap. 100-280, Table top, Auto Turn, Auto Temp, Solid State, Egg Tray $1096. US.
- Auto Humidity Module $384 Prices includes shipping.
Curfew Incubators
- Mod.236: Table Top, Auto, Solid state, Egg Rollers, 50 eggs, About $ 600. Can. landed.
- Mod RX-200: Cabinet, Auto Temp and Humidity, Solid state, Rollers, Backup Heater, Alarm, 110 V, 120 eggs, about $ 1,800. Can landed.
- Mod. RX 201: Cabinet, Auto Temp, Solid state, Egg Rollers, 40 eggs, about $ 900. Can. landed.
One of the oldest incubator manufacturers in the world; in business since 1896. Their dome models are the still air type. Their cabinet incubators appear to be reasonably priced with all of the important features, therefore are an excellent value. HARI now uses the RX-200 with 95% of fertile eggs hatching.
G.Q.F. Manufacturing Co. (Berry Hill Ltd.)
- 1202 Sportsman: Cabinet, Auto, Wafer, 3 lg.trays, 375 eggs,
$280 US.
Economical cabinet incubator used by some large and successful American breeders. Made of wood cabinet which insulates well but is difficult to clean. Fumigation, which may be too dangerous for the hobby breeder, is one of the only ways to penetrate the pores of wood and disinfect. An accurate solid state temperature control, (for instance the one made by Humidaire C.) should perhaps be installed to replace the wafer thermostat.
Grumbach (Swan Creek Supply)
- 8014-Compact S72: Cabinet, Auto, Solid State, Auto. Humidity, 2 trays, 84 eggs, $ 1260 US.
- 8013-BSS 120: Cabinet, Auto, Solid State, Auto. Humidity, 3 trays, 144 eggs, $ 1839 US.
- 8203-BSS 250: Cabinet, Auto, Solid State, Auto Humidity, 6 trays, 288 eggs, $ 2440 US.
Manufactured in Germany and destined to become very popular units in America due to their excellent quality. These highly accurate incubators feature a special rolling device for smooth, slow and assured egg turning. The turning mechanism is adjustible for all sizes of eggs, degree of turn and the number of times turned. All surfaces of the incubating chamber, a poly vinyl coated metal, can be easily disinfected except the water compartment for the source of humidity. Easily read digital instruments are an available option. Rather expensive units.
Rolf C. Hagen, Inc. (Pet Stores)
- Hagen Hatcher Set Up: Composed of one submersible “Thermal Compact” 150 W aquarium heater # A-706, one clear plastic “Critter Pen” # H-302, two small 2.5 gal aquariums # A-1320, $108. suggested retail.
An inexpensive, easy to clean set up, to provide warm moist heat to hatching eggs or act as a brooder (see text for an explanation on their use).
Humidaire Co. (Berry Hill Ltd.)
- Mod. 20: Cabinet, Auto, Solid state, 2 trays, 76 eggs, $ 640 US.
- Mod. 21: Cabinet, Auto, Solid state, 2 lg.trays, 170 eggs, $ 861 US.
- Mod. 50: Drum-type, Auto, Solid state, 3 lg.trays, 540 eggs, $1714 US.
Rather expensive incubators made of redwood cabinets. Humidaire states that their interior wooden walls are sealed for reduced moisture transfer and bacteria resistance. Their tray frames are made of wood and screen with the eggs setting in V-shaped screen inserts. Its advisable to get model 20 & 21 with the solid state temperature control which is an option from the american manufacturer. Berry Hill sells these models with this excellent temperature controller already added. One large American breeder did not have good results with his drum-type incubator and its now collecting dust. Humidaire have been in business since 1931 and provides a long list of organizations who they say are using their equipment.
Kuhl Corporation
- TPT – 120: Dome, Auto, 2x Wafer, 4 trays, 184 eggs, $ 412. US
- AT-600: Cabinet, Auto, 2x Wafer, 20 trays, 920 eggs, $ 1,344. US
Their plastic tray system allows for secure positioning of eggs and complete turning. As with A.B. Incubators, Kuhl egg trays come in different sizes allowing diverse sizes of parrot eggs to be set in the same incubator. The recommended trays for parrot breeders would be WAS-30 for large eggs e.g. macaw, PBT-46 for most eggs e.g. amazon and cockatoo, and Q-80 for smaller eggs e.g. conure and pionus. Their Units are better insulated than those of Marsh Farms. A good value with the large number of different sized eggs they can hold.
Lyon Electric Company, Inc. {was Marsh Farms} (Berry Hill Ltd.)
- Roll-X2: Dome, Auto 1/hr, Solid state, 1 grid, 109 eggs, $ 337 US.
- Profi-I: Cabinet, Auto 1/hr, Solid state, 7 trays, 322 eggs, $ 995 US.
Popular American units now with more accurate and reliable temperature control. Still some concerns over the grid turning system which needs a separate and rather expensive grid for different sizes of eggs. Units are not well insulated and radiant cooling of eggs may occur if room temperature too low. If buying a Roll-X do not buy an old unit as their old thermal wafer temperature control is too inaccurate for parrot eggs. However, Evans (1988) found his Roll-X to have certain spots 30F hotter even with the solid state temperature controls. Excessive vibrations are also a concern yet many of these units are successfully hatching eggs.
- Mod.25: Cabinet, Auto, Solid state, 3 lg.trays, 364 eggs, about $ 900 US.
This is a well built incubator from Spain with good value. However the small manufacturer supplied water tray dries up quickly. We experienced poor hatching when we used the hatching tray at the bottom of this unit. We then developed our Hagen hatcher set up and used the hatching tray in the incubator to hold deep microwave pans which provide water for at least a week. We use the Kuhl egg trays which fit into the Mod. 25 trays. The unit is very accurate in its temperature control and has a turner counter to verify turner operation. Well insulated and easy to clean.
PasReformBV
- C 21: Cabinet, Auto, Solid state, Auto. humidity and cooling, 2 lg.trays, 396 eggs, $ 3,170. US.
- C 41: Cabinet, Auto, Solid state, Auto. humidity and cooling, 4 lg.trays, 792 eggs, $ 3,380. US.
One of the only small incubator lines that has built in cooling with both minimum and maximum temperature and humidity controls. Well insulated cabinet is easy to clean and trays come in various sizes. Water line connection and alarms make for continuous worry free operation. The Mercedes Benz of incubators, made in Holland; excellent quality but at a price. However serious consideration should be given to this line by professional breeders who live in hot and/or humid climates.
Stone House Enterprises
- “R” 1 and “R” 2 fan powered remote auto. humidifiers, $ 175 US.
An add-on to your existing incubator. With this unit higher levels of humidity can be achieved with more consistent levels.
Electronic Weighing Balances
Format:
- Manufacturer (Distributer)
- Model: Maximum capacity, readability,
- Cost: US $.
A&D (Johns Scientific Inc.)
- EK-1200A: 1200 g, 0.1 g, $415.00
- FX-3200: 3100 g, 0.1 g and 0.01 g up to 600 g, $ 1150.00
The EK-1200A has a good capacity for even large birds plus the accuracy to weigh eggs and tiny babies. It has a nice large rectangle pan for steady placement of weighing dishes and is a good value.
Digital Scale (Magic Fan)
- CR 107: 2000 g, 1.0 g, $ 53.00
Economical scale with a capacity that will meet most of the needs of a breeder but too inaccurate for egg weighing.
Ohaus (Technical Marketing Associates Ltd.; Cole-Parmer Co.)
- C305: 300 g, 0.1 g, $ 200.00
- CT600: 600 g, 0.1 g, $ 395.00
- CT1200: 1200 g, 0.1 g, $ 495.00
- E4000D: 4000 g, 0.1 g and 0.01 g up to 400 g, $ 1,195.00
The C305 would be an accurate second scale to compliment the CR 107 above. The CT series pans are rather small and they are more expensive than the similar A&D scale above. The E4000D is a more accurate balance for weighing eggs.
Precision Weighing Balances
Moisture balance, analytical balances, Avian/Ratite/Zoo/Veterinary Models: Ohaus, Acculab
Thermometry and Hygrometry
Cole-Parmer Instrument Company distribute a wide selection of psychrometers, which use the wet bulb method, and digital hygrometers with probes, to measure humidity and digital temperature monitors.
The Cole-Parmer temperature Sentry is a digital monitor plus a high/low alarm with a good accuracy of + 0.2oC (Cat. no. N-08355-32 $185.00). They also carry very accurate glass thermometers; cat no. N-08004-44 having the scale range, 20o-50oC + 0.1oC, best suited for incubation temperatures ($28.70 – length 37 cm with an immersion depth of 7.6 cm). It is important to expose the listed immersion depth of the thermometer to achieve accurate readings.
Manufacturers’ or Distributors Addresses
A.B. Incubators Ltd.
40 Old Market Street
Mendlesham, Suffolk
IP14 5SA UK
Berry Hill Ltd.
75 Burwell Road
St.Thomas, Ontario
N5P 3R5
519-631-0480
Brinsea Products Inc
3670 S Hopkins Avenue
Titusville, FL 32780, USA
Phone: (407) 267-7009
Fax: (407) 267-6090
Supply to Canada, USA, Central & South America
Brinsea Products Ltd
Station Road
Sandford
N. Somerset BS25 5RA
England
Phone:44 (0) 1934 823039
Fax: 44 (0) 1934 820250
This division exports to the rest of the world.
Cole-Parmer Instrument Company
7425 North Oak Ave.
Chicago, Illinois
60648
1-800-323-4340
Curfew Incubators
Southminster Rd.
Althorne, Chelmsford
Essex CM 3 6EN
Fax: 01621 742680
Tel: 01621 741923
G.Q.F. Manufacturing Co.
P.O. Box 1552
Savannah, GA
31498 USA
912-236-0651
Grumbach Brutgerate GmbH
c/o Swan Creek Supply
12240 Spencer Rd.
Saginaw, Michigan 48603 USA
517-642-2716
Humidaire Co.
217 West Wayne St.
New Madison, Ohio
45346 USA
513-996-3001
Jackco Company Ltd.
248 Pennsylvania Ave.
Virginia Beach, VA. 23462 USA
804-499-0999
Johns Scientific Inc.
175 Hanson St.
Toronto, Ont.
M4C 1A7
1-800-268-4410
Kuhl Corporation
P.O. Box 26
Flemington, N.J.
08822-0026 USA
201-782-5696
Lyon Electric Company, Inc.
2765 Main Street
Chula Vista, California
92011 USA
619-585-9900
Magic Fan
2173 Lawrence Ave. E.
Scarborough, Ontario
M1P 2P5
416-752-5128
Masalles Comercial, s.a.
Dosrius 38
Barcelona – 35
Spain
PasReformBV
3130 Verona Ave.
Buford, Georgia
30518 USA
404-945-6403
Precision Weighing Balances
“Electronic Wegighing Balances”
Stone House Enterprises
4298 Bishop Lane
Henderson, KY 42420 USA
502-826-6064
Technical Marketing Associates Ltd.
6695 Millcreek Drive, Unit # 1
Mississauga, Ont.
L5N 9Z9
416-826-7752